Latest News
Successful project completion
Aiming to advance tuberculosis (TB) treatment standards from the current practice of "one-size-fits-...
Interview with Simon Mendelsohn, Clinical Researcher at SATVI
Simon Mendelsohn had an interest in working in the medical field already at an early age, shifting b...
PredictTB Final Meeting
The PredictTB consortium carries out a large-scale clinical study with close to 700 participants in...
PredictTB Learning Board: A new online platform to strengthen capacity building activities
The PredictTB consortium works actively to design and promote training and networking opportunities...
How the PredictTB Student Presentation Session fits into the project’s broader Capacity Building work
Science communication is becoming an increasingly important skill to master for people involved in s...
PredictTB Annual Meeting 2021
The PredictTB consortium held its fourth annual meeting virtually on October 13-14, 2021. The goal o...
MERM: A device to aid patient treatment adherence
Adherence to prescribed treatment is a critical component for tuberculosis (TB) patients’ prospect o...
World Tuberculosis Day 2021: The Clock Is Ticking
Today is World Tuberculosis (TB) Day, which is recognized on March 24 each year. The date marks the...
A look ahead to what 2021 holds for PredictTB
Four years into the project, PredictTB is entering a new phase. In 2021, the project hopes to begin...
Project
PredictTB is a 66-month project financed by a variety of international funders and implemented by American, African, Asian and European partners, that aims to shorten the treatment times of tuberculosis (TB) in drug-sensitive patients through individualized therapy.
Coordinated by Prof. Clifton Barry from the US National Institutes of Health and Prof. Gerhard Walzl from Stellenbosch University in South Africa, the consortium will perform an ambitious phase 2B clinical trial in South Africa and China and develop a set of criteria to reduce TB treatment times using data from scans, assays and cultures to evaluate inflammation and lung pathology, to test for the sustained presence of bacteria, and to determine which patients are eligible for treatment that is much shorter than the current standard of care.
PredictTB Objectives

Trial implementation
PredictTB aims to enroll and follow-up 620 patients with drug sensitive pulmonary TB in a clinical trial to validate candidate biomarkers as well as identify and evaluate new, improved criteria that can identify patients who can be cured with shorter treatment.

Sample collection & storage
The consortium will collect and store biological samples (including serum, whole blood RNA, sputum, saliva, and urine) from patients in Africa and Asia for future biomarker research.
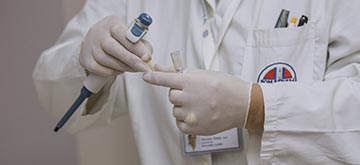
Test development
Project partners in the Netherlands will develop a point-of-care lateral flow device that helps doctors decide which patients are eligible for shortened treatment.
